We are not accepting Valsartan cases at this time.
Quick FactsQuick Facts
Type of Drug: Angiotensin II Receptor Blocker
Brand Names: Diovan, Prexxartan, and others
Drug Used For: High blood pressure and heart failure
Manufacturers Responsible for Contamination: Zhejiang Huahai, Mylan Pharmaceuticals, Inc, Torrent Pharmaceuticals, and others
U.S. Distributors with Lawsuits: Teva Pharmaceuticals Ltd., Solco Healthcare, and others
Issue with Drug: Contains impurities such as NDMA, NDEA, and NMBA
Potential Severe Complications: These impurities are probable human carcinogens
Lawsuit Type: Multidistrict litigation
Lawsuit Status: Active
Valsartan Lawsuit – March 2026 Update
Valsartan Lawsuit – March 2026 Update
Individuals are filing Valsartan lawsuits against Zhejiang Huahai, Mylan Pharmaceuticals, Inc., Teva Pharmaceuticals, Ltd., Solco Healthcare, Torrent Pharmaceuticals, and others because of an increased risk of cancer associated with using the drug. These lawsuits have been consolidated into MDL# 2875 – IN RE: Valsartan, Losartan, and Irbesartan Products Liability Litigation in the United States District Court District of New Jersey. As of March 2, 2026, there are 1,418 pending lawsuits in the federal Valsartan litigation. The presiding judge is Renee Marie Bumb, Chief U.S.D.J. This lawsuit is in mid-stages, with bellwether trials expected to begin in the coming weeks and months. People are continuing to file Valsartan lawsuits today.
Valsartan Lawsuit Timeline & News – March 2026
August 7, 2025: Legal Teams Preparing for Valsartan Bellwether Trials Get Extension
Lawyers for the victims and pharmaceutical companies are preparing for bellwether (test trials) in the Valsartan group litigation. They now have additional time to prepare for these upcoming test trials. Some of the deadlines that were extended include how long they have to get expert reports in order and how long they have to turn in motions and interview witnesses in depositions. These deadlines will help victims’ lawyers strengthen their legal arguments to use during settlement talks or at trial.
July 23, 2025: Valsartan Group Lawsuit Grows as People Recognize Cancer Link
The lawsuit against the companies that made Valsartan, Losartan, and Irbesartan is gaining momentum. July’s total case number grew to 1,342, an uptick of about 20 new claims from the previous month. Multiple batches of Valsartan contained a contaminant (NDMA) that is known to cause cancer. Patients are suing because they discovered that this heart medication may be the reason they developed a range of cancers affecting multiple organs in their bodies. New claims are being added monthly. People can file a claim if they developed cancer after taking Valsartan or if their loved one died because of cancer caused by Valsartan.
July 9, 2025: Preparations for Second Group of Trials Scheduled in Valsartan Federal Lawsuit
The lawyers are preparing for the second grouping of Bellwether trials in the Valsartan lawsuit. The MDL judge has scheduled deadlines for different steps needed to get ready for these trials. For instance, the judge has set a timeline of July 18th to August 20, 2025, for victims to talk to experts who can help strengthen their legal claims. Experts are an important resource in these types of technical cases because they can provide testimony, official opinions, and guidance on how medications like Valsartan can cause cancer. People who are not yet part of this federal MDL can still join, if they meet certain legal requirements.
June 19, 2025: Additional Valsartan Lawsuits Filed in June
Many people who developed cancer after taking Valsartan contaminated with NDMA have joined a group litigation (MDL-2875 IN RE: Valsartan, Losartan, and Irbesartan Products Liability Litigation). There are now 1,325 active lawsuits in the Valsartan litigation, with many people working toward settlements for the pain they have suffered and the costs they have incurred.
June 5, 2025: Test Trials Near in Federal Valsartan Litigation
Many people have filed lawsuits alleging that Valsartan contaminated with NDMA led them to develop cancer. Many of those people have joined a group lawsuit. The judge in that group lawsuit, U.S. District Judge Renee M. Bumb, has set key trial dates for 2025. The first round of bellwether (test) lawsuits will be in October 2025. These lawsuits are often influence the outcomes of other lawsuits in the group litigation. Judge Bumb has also asked attorneys to start preparing for a second round of test trials. A second round of test trials could indicate that the judge expects this litigation to move forward and that settlements could follow.
May 2, 2025: Valsartan Cancer Lawsuit Grows As More People Join the Federal MDL
The lawsuit involving the medication Valsartan grew by 12 cases to 1,322 as of May 1, 2025. Valsartan, also known by the brand name Diovan, was used to treat high blood pressure and cardiac issues. It was recalled after product testing found the drug contained the carcinogen, NDMA. Hundreds of people have filed legal claims to recover compensation after discovering their or their loved one’s cancer may be due to contaminated Valsartan.
April 17, 2025: Valsartan Manufacturer Misses Court Deadline
ZHP, a Chinese company that makes Valsartan, has missed a court deadline for turning over information. ZHP has been named in the large group lawsuit for people harmed by contaminated Valsartan. The judge overseeing the case asked ZHP to turn over documents, including certificates of analysis, material safety data sheets, document retention
policies, and the Chinese “standard policies” for certificates of analysis. Plaintiffs’ attorneys are still waiting for these documents, which will be important in establishing liability in these cases.
April 11, 2025: More People Join Group Lawsuit Against Valsartan Manufacturers
There are currently 1,310 active, pending lawsuits against the makers of Valsartan and other similar drugs. These people have joined the Valsartan, Losartan, and Irbesartan Products Liability Litigation after developing cancer and other serious conditions they say were linked to taking Valsartan. As more people join the litigation, plaintiffs’ lawyers are preparing for the bellwether (test trials), which will occur in September 2025. Preparing arguments for these trials will be important, as these trials will establish important facts for other lawsuits in the group litigation.
March 7, 2025: More People File Lawsuits Against Valsartan Makers Ahead of Test Trials
Attorneys for people who allege contaminants in Valsartan led to their cancer diagnoses are preparing for the first trials in the group litigation. The first trials for the Valsartan multidistrict litigation will happen in 2025. These trials are called bellwether trials and serve as tests for how other lawsuits in the group litigation may play out. Ahead of these trials, more people alleging harm from Valsartan have joined the litigation. As of March 3, there are 1,303 pending lawsuits against Zhejiang Huahai Pharmaceutical Co. and other Valsartan manufacturers. If you took Valsartan and developed certain types of cancer linked to NDMA, you may still be able to file a lawsuit. Surviving family members may also be able to file.
February 25, 2025: Plaintiffs’ Lawyers Say They Have Evidence Contaminants in Valsartan Resulted From Manufacturer’s Practices
In a recent letter to the judge in the Valsartan lawsuit, plaintiff lawyers have indicated that the defendant, ZHP, has turned over important evidence at the end of discovery. Plaintiffs’ lawyers say this evidence shows the presence of key solvents that would have created NDMA and NDEA in Valsartan. The theory of the case is that the manufacturers used cheap processes to make their drugs cheaper and those processes led to the contamination of Valsartan and caused individuals to get cancer.
November 15, 2024: New Bellwether Trial Chosen in Valsartan MDL
The first bellwether trial has been chosen in the Valsartan MDL. Bellwether trials are used to help predict the outcome of similar cases. The parties will meet later this month to determine deadlines for questioning experts and other pre-trial requirements. The trial is expected to begin in September 2025.
October 15, 2024: First Bellwether Trial Rescheduled
The first bellwether trial in the Valsartan MDL was expected to start next month, but the court has agreed to reschedule it for a new date. A new case will be chosen for the bellwether trial in order to better represent a larger portion of the plaintiffs.
October 8, 2024: Plaintiffs in Valsartan MDL Ask for Defense Expert Testimony to Be Dismissed
Plaintiffs in the Valsartan MDL asked the court in a letter dated October 8, 2024, to preclude a defense expert’s testimony from trial. The witness, Dr. Chodosh, was expected to testify about the general causation of cancer and not an agreed-upon question for the jury to consider.
October 1, 2024: Valsartan MDL Grows to 1,270 Cases
Six cases were added to the Valsartan MDL last month, bringing the total number of pending lawsuits to 1,270. Due to the widespread use of the blood pressure drug, it is expected that the number of plaintiffs will grow in the coming weeks and months
On this page:
Valsartan Lawsuit – March 2026 Update
Valsartan Lawsuit Timeline & News – March 2026
What is the Valsartan Lawsuit About?
Why Are People Filing Valsartan Lawsuits?
Whom Are People Valsartan Lawsuits Against?
What Stage is the Valsartan Lawsuit In?
What Is Valsartan and How Does It Work?
Studies Linking Valsartan and Cancer
FDA Warnings and Statements About Valsartan
What Damages Can People Sue Valsartan Manufacturers For?
Who Qualifies to File a Valsartan Lawsuit?
7 Steps to File a Valsartan Lawsuit
What is the Deadline for Filing a Valsartan Lawsuit?
Valsartan Settlements and Awards
What is the Valsartan Lawsuit About?
Valsartan, a drug prescribed to treat high blood pressure and hypertension, was originally manufactured by Novartis and sold under the brand name Diovan. As the initial patent expired, generic versions of the angiotensin II receptor blocker (ARB) flooded the market. Testing of some of these generics detected concerning levels of a cancer-causing agent, N-nitrosodimethylamine (NDMA).
As a result of these findings, the U.S. Food & Drug Administration and European health officials issued a warning to consumers and healthcare providers about a voluntary recall of these drugs. Valsartan patients have reported a number of adverse effects after taking the potentially contaminated drug, including an increased risk of several kinds of cancer. It is believed that millions of people may have been affected due to the widespread use of the medication.
Individuals who took valsartan products contaminated with NDMA, NDEA, and NMBA from 2012 to 2018, mainly made in China, and subsequently diagnosed with cancer, may be entitled to damages.
| Valsartan Lawsuit Information | |
| Lawsuit Name: | In Re: Valsartan, Losartan, and Irbesartan Products Liability Litigation |
| Main Injuries: | Increased risk of cancer |
| Defendants: | Zhejiang Huahai, Mylan Pharmaceuticals, Inc, Teva Pharmaceuticals Ltd., Solco Healthcare, Torrent Pharmaceuticals, and others |
| Mass tort or class action? | Mass Tort |
| MDL Number: | 2875 |
| Pending Cases: | 1,278 |
| Court Name (Venue): | U.S. District Court District of New Jersey |
| Have There Been Settlements? | Yes, although there has not been a global settlement. |
| Active Lawsuit? | Yes |

Why Are People Filing Valsartan Lawsuits?
Individuals are filing valsartan lawsuits nationwide after impurities were detected in multiple lots of the prescription drug. Contaminants included cancer-causing compounds like NDMA, NDEA, and NMBA. Individuals who were diagnosed with cancer after taking affected valsartan drugs may be entitled to damages. Complaints filed against the manufacturers and distributors of these drugs suggest that the companies knew or should have known about the risks associated with using the drug and failed to warn consumers.
Valsartan Lawsuit Spotlight
Gaston Roberts v. ZHP, et al
The case will serve as the first bellwether trial in the federal valsartan MDL out of the District of New Jersey. It involves an Alabama resident who was diagnosed with liver cancer after taking valsartan, which he alleges was contaminated with carcinogenic impurities such as NDMA and NDEA. The trial is expected to start in September 2025.
Whom Are People Valsartan Lawsuits Against?
In 2012, the first generic version of valsartan was approved by the FDA. The market soon became flooded with generic versions of the blood pressure medication. Many distributors of the generic drug received their valsartan from Zhejiang Huahai Pharmaceuticals, a Chinese manufacturer. When tested, it was discovered that valsartan manufactured by Zhejiang Huahai was contaminated with cancer-causing compounds such as NDMA.
Manufacturers and distributors potentially liable in Valsartan lawsuits:
- Zhejiang Huahai Pharmaceuticals
- Teva Pharmaceuticals Ltd
- Solco Healthcare
- Major Pharmaceuticals
- Actavis
- Torrent Pharmaceuticals Limited
- A-S Medication Solutions
- Hetero Labs, Inc.
- Mylan Pharmaceuticals
- AvKARE
- Bryant Rank Prepack
- Preferred Pharmaceuticals
- Camber Pharmaceuticals
- HJ Harkins Company
- Northwind Pharmaceuticals
- NuCare Pharmaceuticals
- Prinston Pharmaceutical
- RemedyRepack Inc.
Manufacturer Profile: Zhejiang Huahai
Zhejiang Huahai is a Chinese pharmaceutical company headquartered in Zhejiang. On November 29, 2018, the U.S. Food and Drug Administration issued a warning letter. The letter indicated that the company had significant deviations from current good manufacturing practice (CGMP) when it came to active pharmaceutical ingredients (API). In particular, the regulatory agency found that the company failed to resolve contamination issues found during earlier testing.
Manufacturer Profile: Mylan Pharmaceuticals, Inc.
Mylan Pharmaceuticals, Inc. is a U.S.-based pharmaceutical company that manufactured a generic version of valsartan. In 2018, the company recalled several lots of valsartan-containing products due to the detection of NDEA (N-Nitrosodiethylamine). Mylan is now part of a new global healthcare company called Viatris.
Manufacturer Profile: Torrent Pharmaceuticals
Torrent Pharmaceuticals, Ltd. is an Indian-based multinational pharmaceutical company that distributed a generic version of valsartan manufactured by the Chinese company Zhejiang Huahai. Torrent issued a voluntary recall of several lots of valsartan after N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) was detected.
Allegations Against Valsartan Manufacturers
Allegations against Valsartan manufacturers and distributors include that the companies knew or should have known of the risks associated with the use of the drug and failed to warn consumers. Legal documents also accuse the companies of failing to ensure their product was safe for consumers and acting negligently.
What Stage is the Valsartan Lawsuit In?
Valsartan lawsuits are in the mid-stages. Over 1,200 claims have been consolidated into a federal MDL out of the District of New Jersey. It is, however, expected that the number of lawsuits could expand considerably over the next months due to the widespread use of the drug. Due to statutes of limitations, which may limit the amount of time a person has to file a lawsuit, individuals diagnosed with cancer after taking valsartan are encouraged to seek legal counsel immediately.
What Is Valsartan and How Does It Work?
Valsartan is an angiotensin II receptor blocker (ARB) used to lower high blood pressure (hypertension) and treat heart failure. It has been shown to reduce the hospitalization risk related to heart failure and improve survival rates after a heart attack. In some cases, it is also used to manage diabetic kidney disease.
The drug was originally marketed by Novartis, receiving FDA approval in 1996 under the brand name Diovan. Diovan contained 80 mg or 160 mg of valsartan. The amount of active ingredient in a generic version of the drug depends on the manufacturer. Inactive ingredients also vary by brand but may include cellulose compounds, povidone, sodium lauryl sulfate, magnesium stearate, iron oxides, gelatin, crospovidone, and titanium dioxide.
Side Effects of Valsartan
There are a number of known side effects of valsartan. These side effects range in severity from relatively minor to severe. Valsartan patients are encouraged to seek the advice of a healthcare professional if they have any new or worsening symptoms.
Common side effects of Valsartan:
- Flu-like symptoms
- Cough
- Fatigue
- Nausea and vomiting
- Headache
- Dizziness
- Joint or muscle pain
- Abdominal pain
- Back pain
- Diarrhea
- High blood potassium (hyperkalemia)
- Low blood pressure (hypotension)
Common side effects are generally mild and temporary in nature. Any persistent or ongoing symptoms should be discussed with a healthcare provider. In most cases, these side effects do not require you to stop taking the medication.
Less-common or more serious side effects of valsartan:
- Bloody or decreased urine
- Irregular heartbeat
- Excessive tiredness
- Paresthesia (tingling or numbness)
- Blurred vision
- Confusion or nervousness
- Difficulty breathing or swallowing
- Swelling of the face, throat, tongue, or extremities
- Unexplained weight gain
- Bone pain
- Increased thirst
- Hives or rash
These serious or rare side effects may require immediate medical attention. At issue in the current lawsuit is the increased risk of cancer associated with the use of the drug. It is believed that the NDMA contamination may have put valsartan patients at a higher risk for developing certain kinds of cancer.
Brand Names of Valsartan
Valsartan is marketed under a number of brand names in single- and multi-ingredient formulations. Alone or in combination with other drugs, the medication helps to relax blood vessels, thereby lowering blood pressure.
Valsartan is sold under the brand names:
- Diovan (single-ingredient)
- Prexxartan (single-ingredient)
- Diovan HCT (multi-ingredient)
- Byvalson/Vyduo (multi-ingredient)
- Entresto (multi-ingredient)
- Exforge (multi-ingredient)
- Exforge HCT (multi-ingredient)
- Valturna (multi-ingredient)
Valsartan Mechanism of Action
Valsartan works by blocking angiotensin II receptors. First, it selectively binds to the angiotensin receptor 1 (ATI), stopping the narrowing or tightening of the blood vessel. Next, it antagonizes the angiotensin II, which improves cardiac function and aldosterone production,
Compared to an ACE inhibitor, valsartan does not rely on a specific production pathway to work. It acts as a complete angiotensin II receptor blocker, not simply the enzymes that produce angiotensin II like an ACE inhibitor. It also does not influence bradykinin levels and may have fewer side effects, such as recurrent cough and angioedema.
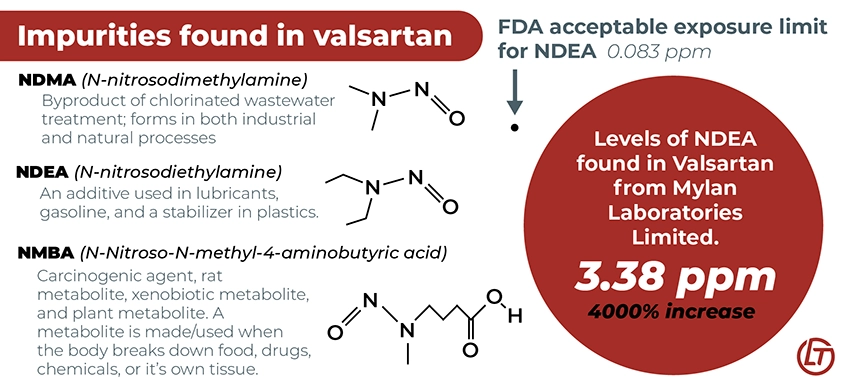
Impurities Found in Valsartan
Testing of generic versions of valsartan, mainly those manufactured by the Chinese pharmaceutical company Zhejiang Huahui, found concerning levels of compounds that may cause cancer, including N-Nitroso-N-methyl-4-aminobutyric acid (NMBA), N-nitrosodimethylamine (NDMA), and N-nitrosodiethylamine (NDEA).
According to the FDA, the acceptable intake level of NDMA and NMBA is 0.3 parts per million (ppm), while it is 0.083 ppm for NDEA. In many cases, the levels far exceeded these limits and even the limits set by the companies' own standards. For instance, valsartan tested from Mylan Laboratories Limited had NDEA levels of 3.38 ppm, over 40x the company’s own standard of 0.08 ppm.
How Did These Impurities Occur?
While additional studies may be needed to determine the exact reason that significant impurities were able to contaminate large batches of valsartan medications, it is believed that the problem may be multifaceted.
Potential causes of impurities in valsartan:
- Pre-existing contamination in raw materials
- Using nitrites in the presence of amines
- Inadequate solvent recovery operations
- Using contaminated water
- A lack of pH or temperature control
- Increased storage time
When investigated by the FDA, it was determined that the manufacturers and distributors of the contaminated valsartan products significantly deviated from current good manufacturing practices (CGMP) for active pharmaceutical ingredients (API).
Cancers Caused By Valsartan
The impurities found in certain generic valsartan products are classified as probable human carcinogens, meaning they could cause cancer. Individuals who took contaminated versions of the drug report higher rates of certain kinds of cancer.
Cancers potentially caused by contaminated valsartan:
- Bladder
- Blood
- Colon
- Colorectal
- Esophageal
- Intestinal (including small intestine)
- Leukemia
- Liver
- Lung
- Multiple Myeloma
- Non-Hodgkin’s Lymphoma
- Prostate
- Rectal
- Stomach or Gastric
The FDA wants to receive reports of injuries and side effects people experience after using a medical device. The FDA Adverse Event Reporting System (FAERS) informs future decisions about drugs. If you developed cancer after taking Valsartan and want to report it, you can learn more about submitting a Medwatch Voluntary Report here
Studies Linking Valsartan and Cancer
Various studies have linked valsartan contaminated with NDMA or nitrosamine and an increased risk of cancer. These studies highlight the need for improved manufacturing practices that eliminate or at least reduce the risk of impurities in widely used medications.
Studies linking contaminated valsartan to an increased risk of cancer:
- Valsartan Case Study: Findings suggest long-term use of drugs with nitrosamine contamination can increase the risk of cancer in a person.
- French Nationwide Study: The nationwide study of 1.4 million valsartan users found a higher risk of liver cancer and melanoma.
- Danish Nationwide Cohort Study: Showed an increased risk for colorectal and uterine cancer in single cancer outcomes. Long-term studies are needed to assess cancer risk.
Important Study: Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications
A 2021 case study published in the International Journal of Environmental Research and Public Health found that in drugs that are used long-term, nitrosamine contamination can increase a person’s risk of developing cancer and pose a concerning risk to public health. The study highlights the need for manufacturing changes and regulatory intervention to prevent contaminated drugs from entering the market.
| Overview: Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications | |
| Description of Study: | Valsartan case study to estimate the cancer risks associated with nitrosamine contamination. |
| Published In: | International Journal of Environmental Research and Public Health |
| Study Authors | Kate Li, Karin Ricker, Feng C. Tsai, et al. |
| Type of Study: | Valsartan Case Study |
| Findings | In drugs that are used long-term, nitrosamine contamination can increase a person’s risk of cancer and pose a serious risk to public health. |
Valsartan Recalls
On July 13, 2018, the U.S. Food and Drug Administration announced the voluntary recall of multiple medicines containing valsartan due to the detection of impurities, including NDMA. NDMA is classified as a probable human carcinogen, meaning it may cause cancer. As noted by the FDA, not all valsartan-containing products were affected by the recall or contamination. The contamination is believed to be the result of manufacturing changes. At the time, the FDA recommended that patients continue to use the medication until they are advised to stop by a healthcare provider or a suitable alternative could be found.
Valsartan products affected by the recall:
- Valsartan manufactured or distributed by Major Pharmaceuticals
- Valsartan and Valsartan/Hydrochlorothiazide (HCTZ) manufactured or distributed by Solco Healthcare.
- Valsartan and Valsartan/Hydrochlorothiazide (HCTZ) manufactured or distributed by Teva Pharmaceuticals Industries, Ltd.
Other manufacturers involved in the recall:
- A-S Medication Solutions LLC
- Aurobindo Pharma
- AvKARE Inc.
- Bryant Ranch Prepack Inc.
- Hetero Labs
- Mylan Pharmaceuticals
- Northwind Pharmaceuticals
- NuCare Pharmaceuticals
- Preferred Pharmaceuticals
- RemedyRepack Inc.
- Rising Pharmaceuticals
- Torrent Pharmaceuticals
Valsartan with Hydrochlorothiazide by Alembic was not affected by the recall. Individuals taking valsartan-containing medicines manufactured or distributed by Solco may be entitled to reimbursement by contacting customerservice@solcohealthcare.com.
FDA Warnings and Statements About Valsartan
The U.S. Food and Drug Administration has issued dozens of updates and press releases related to valsartan contamination. These warnings, dating back to 2018, highlight the serious risks associated with the use of contaminated valsartan. These statements include recall lists, warnings to the manufacturers, and interim limits for NDMA, NMBA, and NDEA levels.
What Damages Can People Sue Valsartan Manufacturers For?
Individuals who were diagnosed with cancer after taking a contaminated version of valsartan may be entitled to damages. Damages may include both economic and non-economic losses. Individuals who lost a loved one to valsartan-related cancer may be able to file a wrongful death claim for additional damages. The amount of damages generally corresponds with the severity of the injuries and the ability to prove losses.
Damages in a Valsartan lawsuit may include:
- Past Medical Expenses: Affected individuals may be entitled to coverage for medical costs and treatment-related expenses from Valsartan-induced conditions.
- Future Treatment Costs: In addition to past medical expenses, plaintiffs may be able to claim damages related to ongoing medical care, long-term treatment, and nursing and rehabilitation expenses.
- Lost Income: A person may be able to claim additional damages if the valsartan-related condition leads to lost wages or reduced income, such as time off work related to cancer treatment.
- Loss of Future Earning Capacity: In some instances, the condition may be debilitating to the point that the person is unable to return to their normal job functions, resulting in a loss of future income.
- Pain and Suffering: Valsartan patients may be able to recover compensation for physical pain and emotional suffering related to their cancer.
- Loss of Companionship or Support: If the injuries resulted in a loss of companionship or support, meaning that the person was unable to contribute to their relationships as they had before the injury, additional compensation may be available.
- Disability or Disfigurement: Any long-term or permanent injuries may entitle a person to compensation related to disability or disfigurement.
- Wrongful Death: If a loved one dies as a result of their valsartan-related cancer, their close family members may be entitled to bring a wrongful death claim.
- Punitive Damages: Under certain circumstances, plaintiffs may be entitled to punitive damages. These damages are designed to help hold the companies accountable for intentional misconduct or gross negligence.
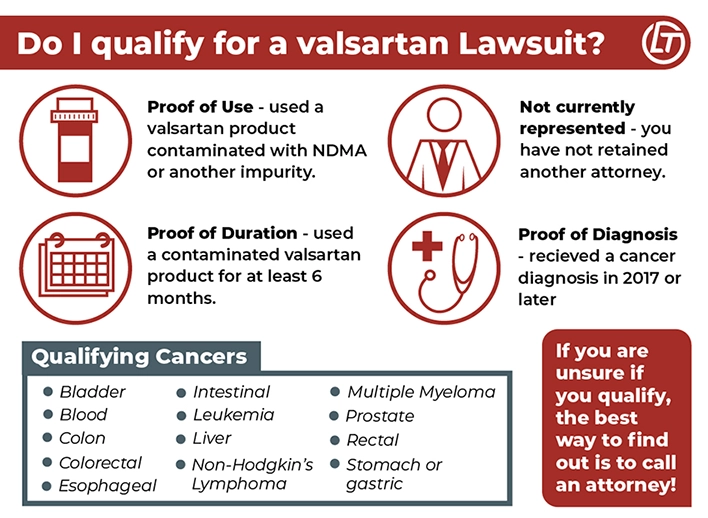
Who Qualifies to File a Valsartan Lawsuit?
In order to file a Valsartan lawsuit, each person has to meet certain eligibility requirements. Our legal partners are focusing on the specific injuries listed below:
- Proof of use: Must have used a valsartan product (single ingredient valsartan or in combination with HCTZ or Amlodipine) contaminated with NDMA or another impurity.
- Proof of duration: Must have used valsartan or valsartan in combination with HCTZ or Amlodipine for at least six months between 2015 and 2018 prior to cancer diagnosis.
- Not currently represented: Cannot be currently represented by an attorney.
- Proof of diagnosis: Proof of cancer diagnosis in 2017 or later.
- Proof of qualifying cancer: Must have been diagnosed with a qualifying cancer.
- Bladder
- Blood
- Colon
- Colorectal
- Esophageal
- Intestinal
- Leukemia
- Liver
- Lung
- Non-Hodgkin’s lymphoma
- Multiple myeloma
- Prostate
- Rectal
- Stomach or gastric
What Proof Do You Need to File a Valsartan Lawsuit?
In order to prove your Valsartan lawsuit, you will need to collect evidence to support your claim. Evidence may include your medical records showing cancer diagnosis and treatment, prescription history establishing when you began taking valsartan, and proof of purchase of a contaminated product.
Evidence of Valsartan Use and Timeline of Use
- Evidence of Product Use:
- Valsartan plaintiffs must be able to prove that they took a valsartan product contaminated with a cancer-causing impurity such as NDMA.
- Must be able to prove that you took valsartan for at least six months prior to the cancer diagnosis.
Evidence About Your Diagnosis and Treatments
- Comprehensive Medical Records: Medical records often play a critical part in valsartan lawsuits. These records should be able to prove the diagnosis of a cancer caused by contaminated valsartan.
- Records may include CT scans, imaging, pathology reports, and other documentation showing your injuries and subsequent treatment.
- Records may include CT scans, imaging, pathology reports, and other documentation showing your injuries and subsequent treatment.
- Prescription History: Your prescription history should show long-term use (at least six months) of valsartan use.
Evidence Showing Your Losses
- Proof of Economic Losses: In order to secure damages you will need to show proof of economic losses such as your medical bills, loss of income, and other out-of-pocket losses.
- Proof of Non-Economic Losses: Affected individuals may also be entitled to non-economic losses such as pain and suffering, loss of enjoyment of life, and disability or disfigurement.
Testimony From You and Your Family
- Proof of Diminished Quality of Life: Personal or witness testimony that can show how your cancer diagnosis affected your quality of life.
7 Steps to File a Valsartan Lawsuit
To file a successful valsartan lawsuit, you need to follow certain steps. The first step is to consult with an experienced attorney. An attorney can help determine whether you meet the eligibility requirements necessary to file a claim and provide guidance throughout the legal process.
These are the 7 steps for filing a Valsartan lawsuit:
- Consult with a Qualified Attorney: The first step in a Valsartan lawsuit is to consult with an experienced attorney. An attorney can help review the claim to determine whether you meet the necessary requirements to file a lawsuit. They will also walk you through the legal process.
- Obtain the Necessary Evidence: Your legal representative will help identify what evidence is necessary to file a lawsuit against the manufacturer or distributor of valsartan. Documents necessary to substantiate your claim include medical records, prescription history, pay stubs, and proof of out-of-pocket losses.
- File the Claim: With the necessary evidence collected, your attorney can file the claim. Prior to filing, your attorney will ensure that your case is filed within the statute of limitations and in the appropriate courthouse. Depending on the circumstances of the case, your case may be consolidated with existing lawsuits in an MDL.
- Enter the Discovery Phase: After the case is filed, the lawsuit may enter the discovery phase, where both parties gather and exchange information.
- Negotiate for a Settlement: Throughout the case, your attorney may negotiate with the other party (generally the manufacturer or distributor of valsartan) to obtain a favorable settlement.
- Set for Trial: If a favorable settlement cannot be reached, the matter may be set for trial. At trial, both parties present their cases, and a judge or jury decides the outcome.
- Post-Trial/Settlement: If a favorable settlement is negotiated or you are awarded damages by a judge or jury, your attorney will help to secure your payout as quickly as possible.
What to Expect When Filing a Valsartan Lawsuit
The best way to determine what to expect when filing a Valsartan lawsuit is to consult with an experienced attorney. An attorney can help you navigate the process. It is important to know that the legal process can be lengthy, but accepting a settlement may expedite a resolution. Additionally, attorneys generally accept cases on a contingency fee basis, meaning there are no upfront fees. Finally, the compensation you may receive will depend on a number of factors, including the severity of the injury and the ability to prove the connection between your cancer diagnosis and valsartan use.
What is the Deadline for Filing a Valsartan Lawsuit?
In general, there is a two-year statute of limitation for valsartan lawsuits. However, since there is no federal statute of limitations, the deadline for filing may vary by state. Due to the short amount of time you have to file a lawsuit, it is essential to contact an attorney as soon as possible. An attorney can help ensure that all legal requirements are met.
The statute of limitations starts from the date of diagnosis in most cases, although there are some exceptions. The date may also begin from the date of discovery or the date you discovered the connection between your valsartan use and your cancer diagnosis.
If the cancer developed from valsartan contaminated with NDMA, you may be eligible to join the valsartan MDL. The complexities of these legal deadlines make it imperative to discuss your case with an attorney as soon as possible.
Valsartan Settlements and Awards
Valsartan cases are expected to settle for between $100,000 and over $400,000. Although there is not currently a global settlement, it is expected that there will be a tier system for Valsartan lawsuit payouts based on factors such as the severity of the injury, cancer type, age, and proof of Valsartan use.
Top-tier cases or those with the most severe injuries have the potential to settle for over $400,000. Middle-tier cases may settle for between $200,000 and $300,000, while bottom-tier cases may settle for less than $100,000.
Average Settlement Amounts
The estimated average payout is expected to be between $150,000 and $200,000. However, there is the potential for some cases to settle for significantly more depending on the severity of the injuries and other factors.
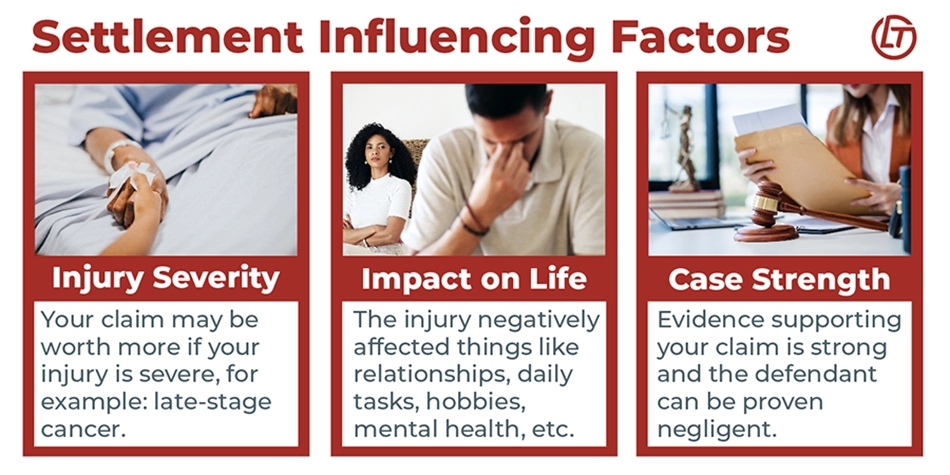
Factors Affecting the Value of a Valsartan Lawsuit
Several factors may influence the value of a Valsartan lawsuit. An attorney can help to determine how much your case is worth.
Factors that may influence your valsartan settlement or jury award:
- Injury Severity: The more severe the injury, the more your claim may be worth. For example, a person diagnosed with a later-stage cancer with limited treatment options may be entitled to a higher settlement.
- Impact on Quality of Life: If the injury diminished your quality of life, you may be entitled to additional compensation.
- Proof of Negligence: If the defendant (the manufacturer or distributor of valsartan) was grossly negligent or engaged in intentional misconduct, you may be entitled to punitive damages.
- Strength of Claim: The strength of the claim may improve the chances of receiving a larger settlement or payout.
Learn More About Valsartan Lawsuits From Our Legal Team
Research confirms that contaminated valsartan may increase the risk of developing certain kinds of cancer. If you took a generic version of the drug and were subsequently diagnosed with cancer, you may qualify for compensation. It is important to contact an attorney as soon as possible to help you navigate the legal process. Our legal team is well-versed in valsartan lawsuits and will work to hold pharmaceutical companies accountable for their negligence or wrongdoing.
Get A FREE Case Review
Contact Us TodayFAQs
Certain versions of valsartan-containing products were recalled after impurities were detected. The impurities included potentially cancer-causing compounds such as NDMA, NMBA, and NDEA.
Several lots of valsartan were recalled after testing detected impurities such as NDMA. NDMA is listed as a potential human carcinogen, which means that it may cause cancer after exposure.
Valsartan lawsuits have been filed nationwide. Over 1,200 cases have been consolidated in multidistrict litigation out of the District of New Jersey.
Qualifications for filing a Valsartan lawsuit include proof of use of a version of the drug contaminated with NDMA or another impurity. Additionally, plaintiffs must be able to show that they used the drug for at least six months prior to a qualifying cancer diagnosis.
The Valsartan MDL is in its mid-stages, with the first bellwether trial expected to start in September 2025. Potential plaintiffs are encouraged to seek legal representation as soon as possible to ensure timely filing of their lawsuit.
Valsartan settlements are expected to happen in the coming months. If a global settlement is accepted, it will likely involve a tiered payout system. Top-tier cases will receive larger settlements due to the severity of the plaintiff's injuries.
Valsartan lawsuits are expected to payout between $150,000 and $200,000. However, there is the potential for some lawsuits to payout $400,000 or more due to the severity of the injuries.
Contact Us Today
"*" indicates required fields